Neuralink Begins Human Trials: What It Means for the Future
Elon Musk’s Neuralink Begins First Human Trials
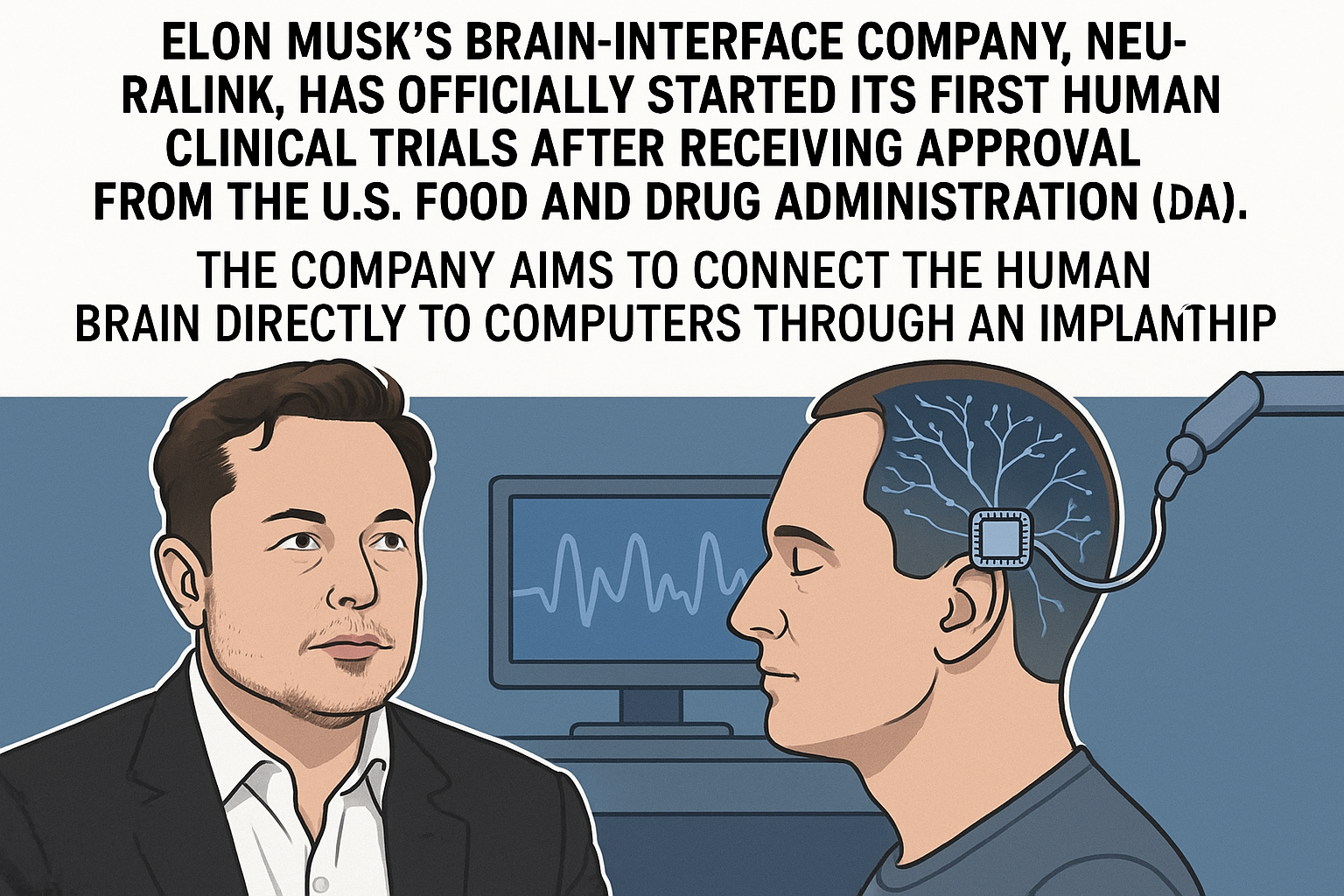
Elon Musk’s brain-interface company, Neuralink, has officially started its first human clinical trials after receiving approval from the U.S. Food and Drug Administration (FDA). The company aims to connect the human brain directly to computers through an implanted chip.
But what does this mean for the future of healthcare, artificial intelligence, and the human mind? In this post, we’ll break down the latest updates, expert reactions, and potential risks associated with this revolutionary technology.
What Is Neuralink Trying to Achieve?
Neuralink’s long-term goal is to help people with neurological disorders, such as ALS and spinal cord injuries, regain control of their bodies. According to the company, the initial trial — named “PRIME Study” — focuses on individuals who are quadriplegic due to cervical spinal cord injury or amyotrophic lateral sclerosis (ALS).
Moreover, the chip is designed to wirelessly record and transmit brain signals, allowing users to control digital devices like phones or computers directly through thought. If successful, it could completely transform human-computer interaction.
Public Reaction and Ethical Concerns
Not surprisingly, the public’s reaction is mixed. While many tech enthusiasts are excited about the possibilities, scientists and ethicists are raising concerns. For instance, some worry about long-term side effects and the implications of corporations gaining access to human neural data.
Meanwhile, various organizations, including the Neuroethics Working Group, have called for stricter regulation. Additionally, the World Health Organization (WHO) has highlighted the need for global standards around brain-machine interfaces.
What Comes Next?
The PRIME Study will take six years to complete. If it proves successful, Neuralink may expand its application beyond medical use into areas such as memory enhancement and even digital telepathy.
However, until peer-reviewed results are published, skepticism remains. Regulatory bodies will continue to monitor the safety and effectiveness of the implant. The company is also expected to publish updates on trial participants and progress on its official blog.
Internal & External Links
Tags: Elon Musk, Neuralink, Brain Chip, Human Trials, Tech News, AI, Bioethics, Medical Technology, FDA Approval
Sources: